How Intelligent ECAD-MCAD Tools Facilitate Rapid Medical Device Prototyping

Many electronic medical devices incorporate mechanical form factors which restrict the size, space, and placement of components in the PCB. In the not-too-distant past, mechanical prototyping relied heavily on prototyped electrical and mechanical assemblies to ensure form and fit inside the device enclosure or in the intended deployment environment. However, today’s MCAD tools have helped to eliminate mechanical prototyping spins while enabling more exotic form factors for medical electronics.
Nowadays, ECAD tools are catching up with MCAD software, enabling the two disciplines to collaborate on the design of complex medical electronics. This means the ECAD-MCAD bridge can help accelerate medical innovation and prevent costly design errors.
Medical Device Prototyping: Who’s Responsible?
ECAD and MCAD roles are clearly defined but require collaboration to ensure electrical designs will fit within mechanical designs. In the past, file packages were sent back-and-forth between ECAD and MCAD software users, while today, cloud services are enabling the link across engineering disciplines.
Translate Electrical to Mechanical
Mechanical engineers rely on real-time visibility of PCB layouts to create, for example, compact packets for devices small enough to function within the body or that conform to intricate anatomical structures. Devices may be flexible, implantable modules or have wearable housings.
This ensures that the electronics fit precisely within the mechanical enclosure specification, support patient-safe ergonomics (as per the mechanical layout), and maintain performance without compromising sterility, mechanical integrity, or biocompatibility.
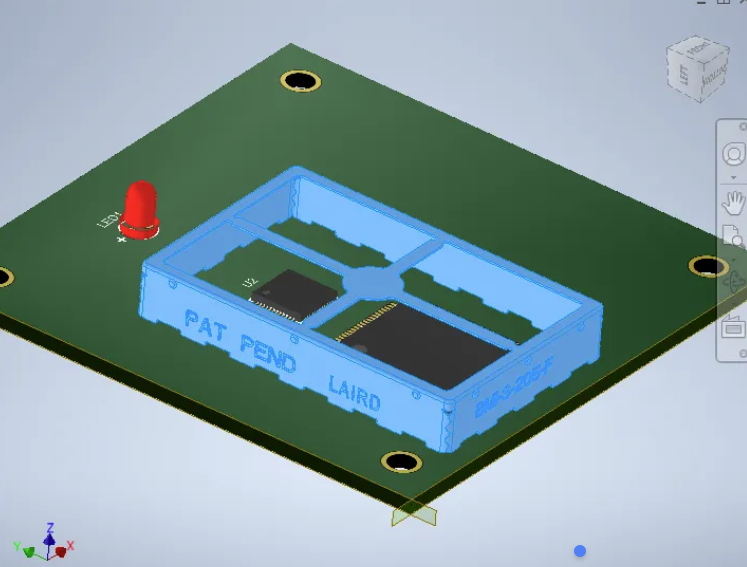
Translate Mechanical to Electrical
Likewise, the understanding among electrical teams of the mechanical implications of their PCBs (material choices, part placement, and proximity risks) is vital for ensuring medical devices can achieve their usability and treatment objectives.
This includes electrical engineers factoring in how enclosure features such as mounting bosses, connector orientation, or thermal vents create constraints on the PCB form factor and component placement. If, for instance, a mechanical enclosure has a tightly curved surface or low profile, the PCB layout in ECAD needs to have keepouts defined for excessively tall components.
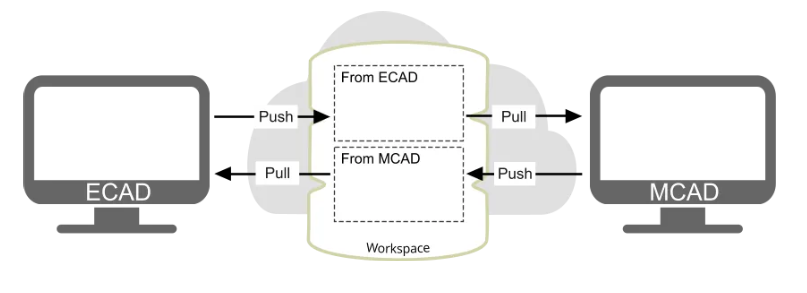
The Risk of Poor ECAD-MCAD Design Integration
Defective designs can have adverse effects (i.e. cause pain or discomfort to patients, or disrupt their care).
Inadequate integration between ECAD and MCAD domains often leads to mechanical-electrical mismatches, such as misaligned connectors, incorrect board shapes, or thermal issues that were not accounted for early in the design phase. These errors can delay product development, increase prototyping costs, or result in non-compliance with regulatory standards like FDA 21 CFR Part 820 or ISO 13485.
In terms of medical devices, small design oversights can lead to serious implications. For instance, a misfitted PCB inside a diagnostics device may cause intermittent electrical faults, compromising the accuracy of test results. In wearable or implantable devices, such integration issues could affect patient comfort or lead to device failure in critical scenarios.
Poor synergy between ECAD and MCAD tools also hampers traceability, a key requirement in regulated environments and, in terms of medical, a core source of insight. Without a seamless workflow, it becomes difficult to maintain consistent documentation, track design changes, and demonstrate risk mitigation during audits.
Investing in robust ECAD-MCAD integration tools and workflows is not just a matter of efficiency but also a fundamental requirement for crafting safe, effective, and compliant medical devices.
ECAD-MCAD Integration in Example Medical Device Prototypes
Here are some of the ways in which ECAD-MCAD integration aids the development of specific medical items.
Pacemakers and Implantable Cardioverter-Defibrillators
- ECAD: 3D models of PCBs are shared so that enclosure designs can route internal wiring and connectors and avoid mechanical interferences. This is crucial for these devices with titanium casings. Its conductivity means greater emphasis on component spacing for efficient thermal dynamics.
- MCAD: Changes to the geometry of devices are fed back to PCB designers to ensure relevant updates are made in order to prevent component collision and lead strain. Real-time coordination aids both teams when it comes to adjusting their designs for thermal efficiency and product longevity.
Ventricular Assist Devices
- ECAD: Electrical engineers can communicate circuitry requirements for motor control, sensors, and controllers as they are built into mechanical models of the pump. This ensures that cabling, connectors, and sensors fit alongside rotating impellers and bearings.
- MCAD: Mechanical teams must communicate physical stress factors and thermal constraints of housing designs. With ECAD-MCAD integration, these updates can be translated seamlessly into PCB workflows for signal path redesign or thermal safety margins.
Ventilators and Diaphragm Pacers
- ECAD: Real-time transfer of PCB outline, sensor, and actuator placement helps mechanical teams route tubing, valves, and motors in the correct physical footprints of more and more compact prototypes.
- MCAD: Mechanical constraints, such as that of airflow channel position or frame supports, feed back to layout engineers. They position sensors and components around these constraints, having been made aware of alterations in advance or real time.
External Diagnostic and Imaging Equipment (ECG, Ultrasound)
- ECAD: Complex circuit boards, including analog front-end and processor modules, are aligned within probe housing or chassis to ensure proper connector positioning and thermal clearance.
- MCAD: Thermal design of mechanical enclosures or internal airflow paths inform ECAD about placement of heat-generating components, guiding suitable placement for cooling or shielding.

ECAD-MCAD Tools to Improve Medical Device Prototyping
Choosing the right ECAD-MCAD integration tool gives teams the chance to eliminate electrical and mechanical prototyping spins which would normally be used for testing form and fit. The most effective solutions are those that support seamless data exchange between electric and mechanical design platforms.
Key capabilities to look for include:
- Bidirectional Data Synchronization: The ability to update board shapes, component placement, and mechanical constraints without manual file transfers.
- Cloud-Based Collaboration: Cloud-enabled platforms can help distributed or cross-functional teams work together more efficiently, especially for smaller design teams working on tight timelines.
- Simulation and Testing Features: Built-in tools for stress testing, thermal simulation, and generative design can support ergonomic and functional optimization early in the prototyping process.
- Supporting for Injection-Molded and Wearable Designs: Tools that handle specific needs like modeling flexible housings, routing internal cabling, or testing enclosure durability are particularly valuable for medical applications.
- Enterprise-Scale Integration: For larger medical device firms, integration with enterprise-level systems can streamline version control, compliance tracking, and design traceability across global teams.
As devices become smaller, smarter, and more deeply embedded in critical patient care, the alignment between electrical and mechanical design grows in importance. Investing in integrated tools and collaborative digital environments helps teams visualize prototypes more effectively: they can iterate faster, reduce risk, and bring life-saving innovations to market with greater confidence.